do i include lone pairs in 3d drawings
9.2: The VSEPR Model
- Page ID
- 21752
Learning Objectives
- To use the VSEPR model to predict molecular geometries.
- To predict whether a molecule has a dipole moment.
The Lewis electron-pair approach tin be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms accept lone pairs of electrons. This approach gives no data about the actual organization of atoms in space, however. We continue our give-and-take of structure and bonding by introducing the valence-beat out electron-pair repulsion (VSEPR) model (pronounced "vesper"), which can be used to predict the shapes of many molecules and polyatomic ions. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
The VSEPR Model
The VSEPR model can predict the structure of virtually any molecule or polyatomic ion in which the central atom is a nonmetal, likewise equally the structures of many molecules and polyatomic ions with a cardinal metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore prefer the geometry that places electron pairs as far autonomously from each other as possible. This theory is very simplistic and does non account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair arroyo.
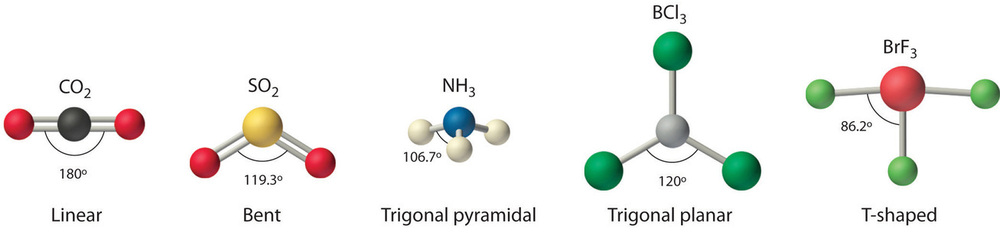
Nosotros can utilize the VSEPR model to predict the geometry of most polyatomic molecules and ions past focusing only on the number of electron pairs around the central cantlet, ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bail, a double bond, a triple bail, a solitary pair of electrons, or even a unmarried unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.east., the one with the lowest free energy) is the one that minimizes repulsions. Groups are positioned around the central cantlet in a way that produces the molecular structure with the everyman free energy, as illustrated in Figures \(\PageIndex{ane}\) and \(\PageIndex{two}\).
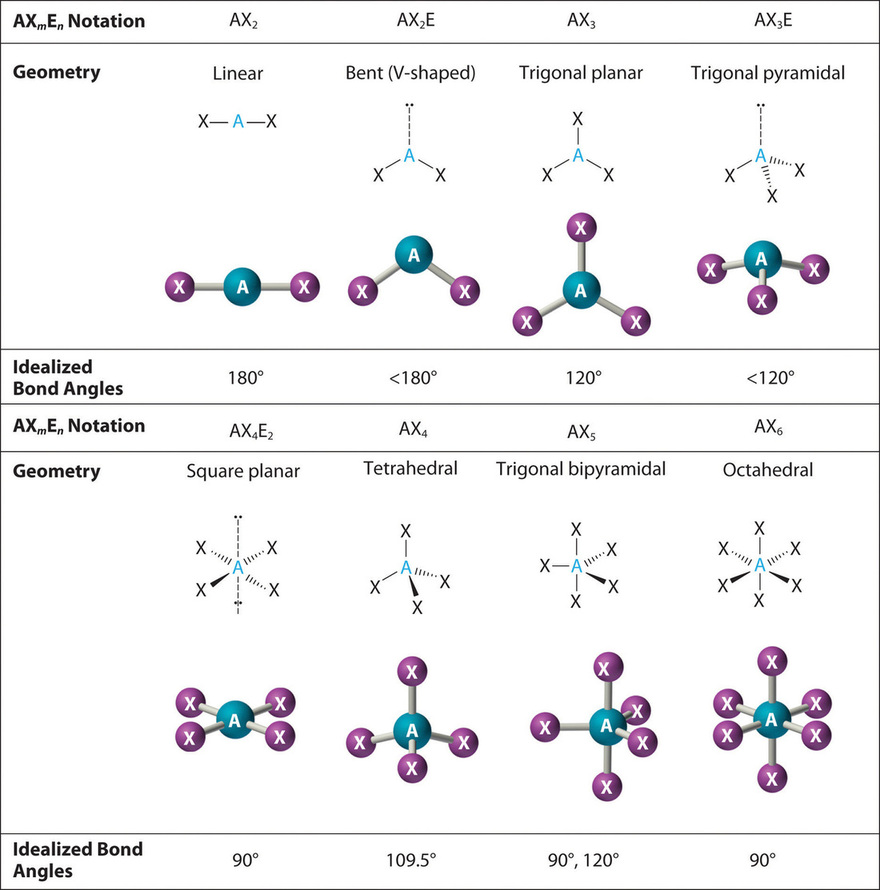
In the VSEPR model, the molecule or polyatomic ion is given an AX m E n designation, where A is the fundamental atom, 10 is a bonded atom, Eastward is a nonbonding valence electron group (commonly a solitary pair of electrons), and grand and n are integers. Each group around the central atom is designated as a bonding pair (BP) or lonely (nonbonding) pair (LP). From the BP and LP interactions nosotros can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. Using this information, we tin can describe the molecular geometry, the arrangement of the bonded atoms in a molecule or polyatomic ion.
VESPR Produce to predict Molecular geometry
This VESPR procedure is summarized equally follows:
- Draw the Lewis electron structure of the molecule or polyatomic ion.
- Determine the electron group organisation around the cardinal cantlet that minimizes repulsions.
- Assign an AX chiliad E n designation; and then place the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bail angles.
- Describe the molecular geometry.
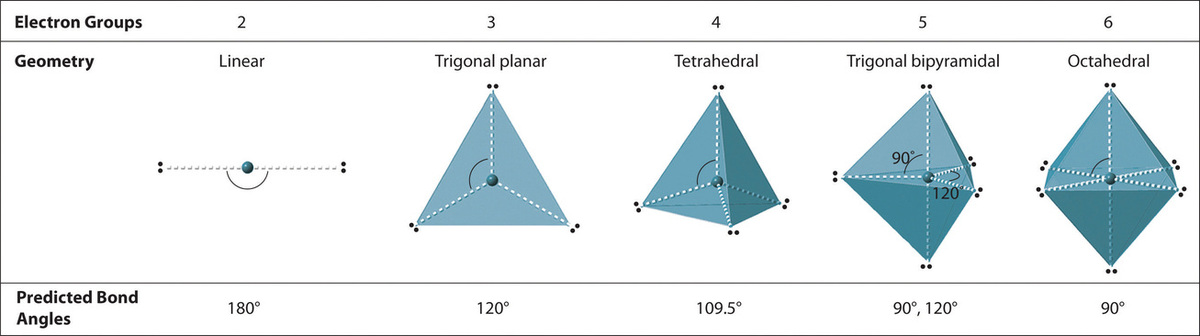
We will illustrate the use of this process with several examples, beginning with atoms with two electron groups. In our discussion we volition refer to Effigy \(\PageIndex{2}\) and Effigy \(\PageIndex{3}\), which summarize the common molecular geometries and idealized bail angles of molecules and ions with two to 6 electron groups.
Two Electron Groups
Our first instance is a molecule with two bonded atoms and no lone pairs of electrons, \(BeH_2\).
AX2 Molecules: BeH2
ane. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is

3. Both groups around the central atom are bonding pairs (BP). Thus BeH2 is designated every bit AXtwo.
4. From Figure \(\PageIndex{iii}\) we see that with two bonding pairs, the molecular geometry that minimizes repulsions in BeHtwo is linear.
AX2 Molecules: COii
ane. The central atom, carbon, contributes four valence electrons, and each oxygen atom contributes half dozen. The Lewis electron structure is

2. The carbon cantlet forms two double bonds. Each double bond is a grouping, so in that location are ii electron groups effectually the primal atom. Similar BeHtwo, the organization that minimizes repulsions places the groups 180° autonomously.
3. Once over again, both groups effectually the key cantlet are bonding pairs (BP), so CO2 is designated every bit AX2.
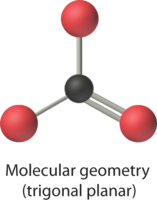
4. VSEPR simply recognizes groups effectually the central atom. Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. With 2 bonding pairs on the key atom and no lone pairs, the molecular geometry of CO2 is linear (Figure \(\PageIndex{3}\)). The construction of \(\ce{CO2}\) is shown in Figure \(\PageIndex{1}\).
Three Electron Groups
AX3 Molecules: BCliii
1. The central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. The Lewis electron construction is
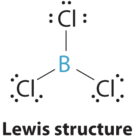
three. All electron groups are bonding pairs (BP), so the construction is designated equally AXiii.
4. From Figure \(\PageIndex{3}\) we see that with iii bonding pairs effectually the primal cantlet, the molecular geometry of BClthree is trigonal planar, every bit shown in Figure \(\PageIndex{two}\).
AX3 Molecules: COthree ii −
1. The central atom, carbon, has four valence electrons, and each oxygen cantlet has six valence electrons. As you learned previously, the Lewis electron structure of one of iii resonance forms is represented as

3. All electron groups are bonding pairs (BP). With 3 bonding groups around the fundamental atom, the construction is designated every bit AXthree.
4. We see from Effigy \(\PageIndex{3}\) that the molecular geometry of CO3 two − is trigonal planar with bond angles of 120°.
In our adjacent instance we encounter the effects of lone pairs and multiple bonds on molecular geometry for the first fourth dimension.
AX2Eastward Molecules: Then2
i. The cardinal atom, sulfur, has 6 valence electrons, as does each oxygen atom. With 18 valence electrons, the Lewis electron structure is shown beneath.
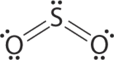
3. At that place are two bonding pairs and one lone pair, then the construction is designated equally AX2E. This designation has a total of three electron pairs, two X and one Due east. Considering a alone pair is not shared by two nuclei, it occupies more infinite well-nigh the fundamental atom than a bonding pair (Figure \(\PageIndex{4}\)). Thus bonding pairs and lone pairs repel each other electrostatically in the lodge BP–BP < LP–BP < LP–LP. In So2, we have 1 BP–BP interaction and two LP–BP interactions.
4. The molecular geometry is described just by the positions of the nuclei, not by the positions of the lone pairs. Thus with two nuclei and 1 lonely pair the shape is bent, or Five shaped, which tin can be viewed every bit a trigonal planar arrangement with a missing vertex (Figures \(\PageIndex{2}\) and \(\PageIndex{three}\)). The O-S-O bond angle is expected to be less than 120° because of the extra space taken up by the lonely pair.

Equally with Soii, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom.
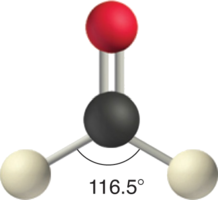
Like lone pairs of electrons, multiple bonds occupy more than space effectually the primal atom than a single bond, which can cause other bond angles to be somewhat smaller than expected. This is because a multiple bond has a higher electron density than a single bond, and so its electrons occupy more space than those of a single bail. For instance, in a molecule such as CH2O (AX3), whose structure is shown below, the double bail repels the unmarried bonds more than strongly than the unmarried bonds repel each other. This causes a deviation from ideal geometry (an H–C–H bond angle of 116.5° rather than 120°).
Four Electron Groups
One of the limitations of Lewis structures is that they depict molecules and ions in simply 2 dimensions. With four electron groups, nosotros must acquire to show molecules and ions in three dimensions.
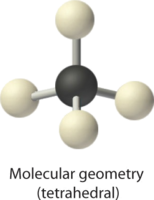
AX4 Molecules: CH4
ane. The central atom, carbon, contributes four valence electrons, and each hydrogen atom has one valence electron, and then the total Lewis electron structure is
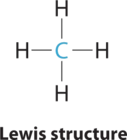
two. At that place are four electron groups around the key cantlet. Every bit shown in Figure \(\PageIndex{2}\), repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 109.v°.
3. All electron groups are bonding pairs, so the structure is designated as AXiv.
4. With four bonding pairs, the molecular geometry of marsh gas is tetrahedral (Figure \(\PageIndex{3}\)).
AX3E Molecules: NH3
1. In ammonia, the central atom, nitrogen, has v valence electrons and each hydrogen donates ane valence electron, producing the Lewis electron structure
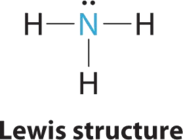
ii. There are four electron groups around nitrogen, iii bonding pairs and one solitary pair. Repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron.
3. With three bonding pairs and one lonely pair, the construction is designated as AX3E. This designation has a full of four electron pairs, three X and one Due east. We expect the LP–BP interactions to cause the bonding pair angles to deviate significantly from the angles of a perfect tetrahedron.
4. There are three nuclei and 1 lone pair, so the molecular geometry is trigonal pyramidal. In essence, this is a tetrahedron with a vertex missing (Figure \(\PageIndex{3}\)). However, the H–Due north–H bond angles are less than the ideal angle of 109.5° because of LP–BP repulsions (Figure \(\PageIndex{3}\) and Figure \(\PageIndex{4}\)).
AXiiEii Molecules: H2O
one. Oxygen has six valence electrons and each hydrogen has one valence electron, producing the Lewis electron structure
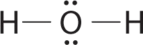
3. With two bonding pairs and two lonely pairs, the structure is designated as AX2E2 with a full of 4 electron pairs. Due to LP–LP, LP–BP, and BP–BP interactions, nosotros expect a pregnant departure from idealized tetrahedral angles.
4. With two hydrogen atoms and two lone pairs of electrons, the structure has pregnant lone pair interactions. There are ii nuclei nigh the central atom, so the molecular shape is bent, or Five shaped, with an H–O–H angle that is even less than the H–Northward–H angles in NH3, equally we would expect because of the presence of ii alone pairs of electrons on the fundamental atom rather than one. This molecular shape is essentially a tetrahedron with two missing vertices.

Five Electron Groups
In previous examples it did non matter where we placed the electron groups because all positions were equivalent. In some cases, however, the positions are not equivalent. Nosotros encounter this situation for the showtime time with five electron groups.
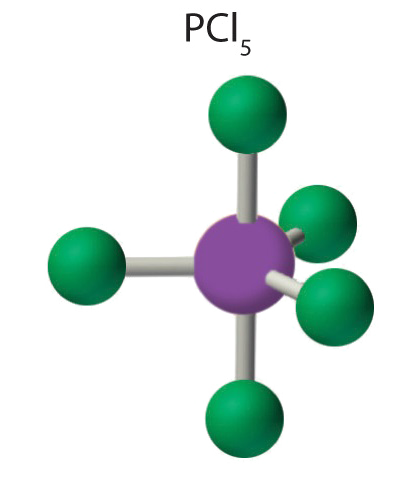
AX5 Molecules: PCl5
1. Phosphorus has v valence electrons and each chlorine has seven valence electrons, and so the Lewis electron structure of PCl5 is
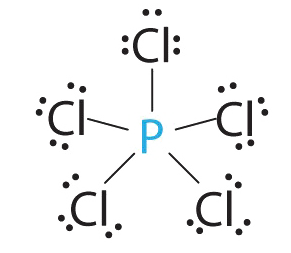
iii. All electron groups are bonding pairs, so the construction is designated as AX5. There are no lone pair interactions.
4. The molecular geometry of PClfive is trigonal bipyramidal, as shown in Figure \(\PageIndex{3}\). The molecule has three atoms in a plane in equatorial positions and 2 atoms in a higher place and beneath the plane in axial positions. The three equatorial positions are separated by 120° from one another, and the two axial positions are at xc° to the equatorial plane. The centric and equatorial positions are not chemically equivalent, equally we volition come across in our next instance.
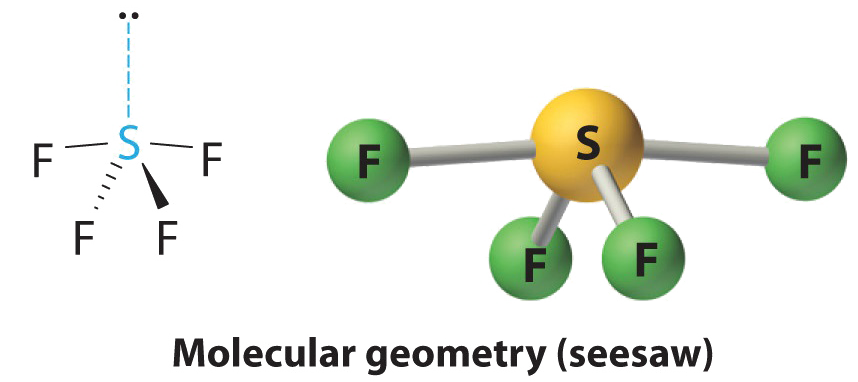
AXivEast Molecules: SF4
1. The sulfur atom has six valence electrons and each fluorine has seven valence electrons, and so the Lewis electron structure is

With an expanded valence, this species is an exception to the octet rule.
two. There are 5 groups around sulfur, four bonding pairs and one lone pair. With five electron groups, the lowest energy organisation is a trigonal bipyramid, equally shown in Figure \(\PageIndex{ii}\).
3. We designate SF4 every bit AXfourDue east; information technology has a full of five electron pairs. However, considering the axial and equatorial positions are non chemically equivalent, where practice we place the lone pair? If we place the lonely pair in the centric position, nosotros have three LP–BP repulsions at ninety°. If we identify information technology in the equatorial position, we have two 90° LP–BP repulsions at 90°. With fewer xc° LP–BP repulsions, nosotros can predict that the structure with the lone pair of electrons in the equatorial position is more than stable than the one with the lone pair in the axial position. Nosotros also wait a deviation from ideal geometry because a lone pair of electrons occupies more space than a bonding pair.
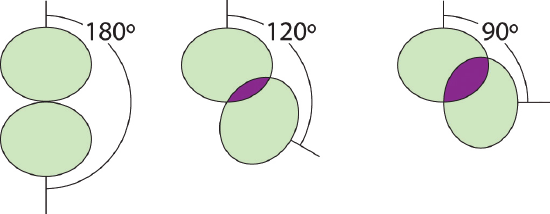
At ninety°, the two electron pairs share a relatively large region of space, which leads to strong repulsive electron–electron interactions.
four. With four nuclei and one alone pair of electrons, the molecular construction is based on a trigonal bipyramid with a missing equatorial vertex; information technology is described every bit a seesaw. The Faxial–Due south–Faxial angle is 173° rather than 180° because of the lone pair of electrons in the equatorial plane.
AX3Eastward2 Molecules: BrFthree
one. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is
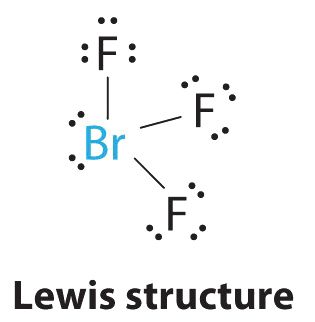
Once once again, we have a compound that is an exception to the octet dominion.
two. At that place are v groups effectually the central atom, three bonding pairs and two lone pairs. We over again direct the groups toward the vertices of a trigonal bipyramid.
iii. With 3 bonding pairs and ii alone pairs, the structural designation is AXthreeE2 with a total of 5 electron pairs. Because the centric and equatorial positions are not equivalent, nosotros must decide how to arrange the groups to minimize repulsions. If we identify both lone pairs in the centric positions, we have vi LP–BP repulsions at 90°. If both are in the equatorial positions, we have four LP–BP repulsions at 90°. If one solitary pair is axial and the other equatorial, we take i LP–LP repulsion at ninety° and three LP–BP repulsions at 90°:
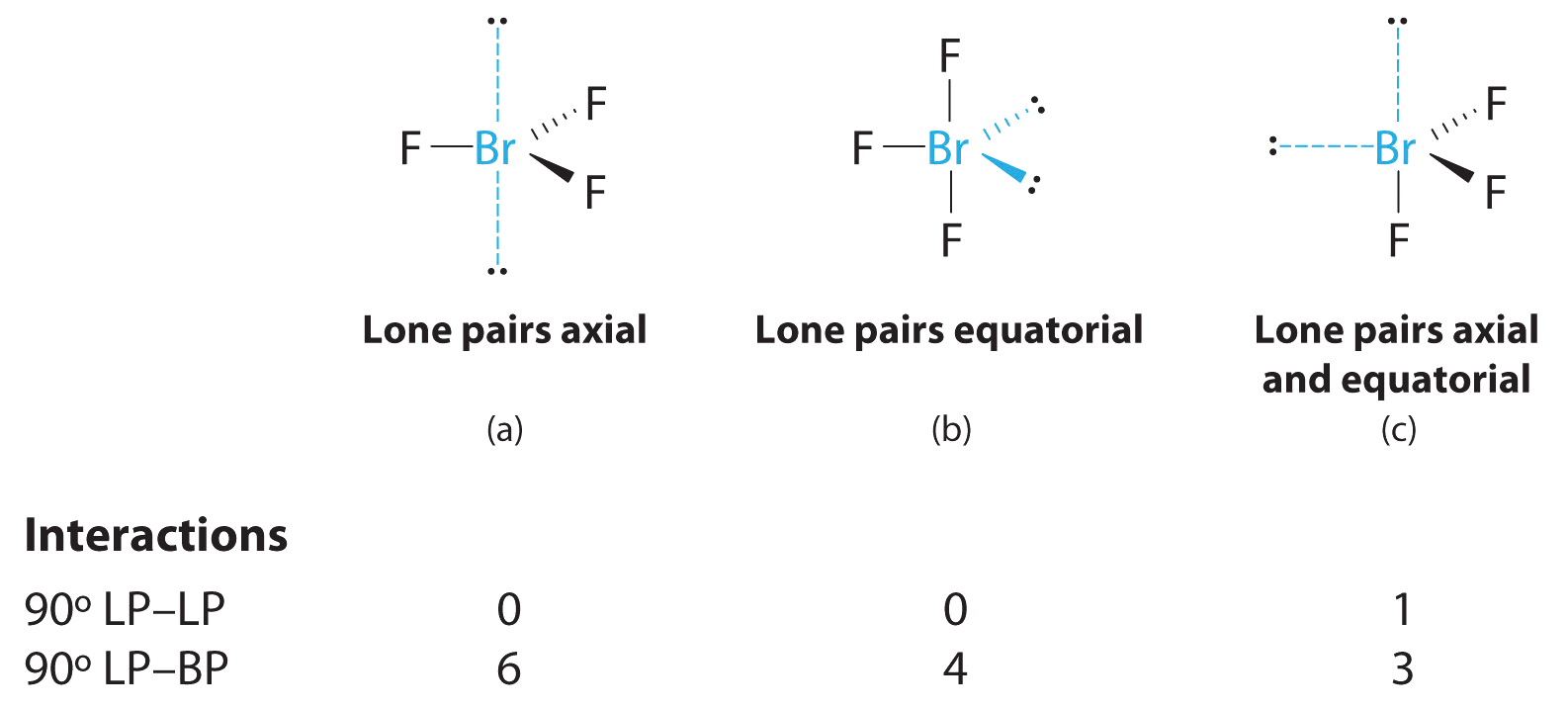
Construction (c) tin be eliminated because it has a LP–LP interaction at 90°. Construction (b), with fewer LP–BP repulsions at xc° than (a), is lower in energy. However, nosotros predict a deviation in bond angles because of the presence of the two lone pairs of electrons.
4. The three nuclei in BrF3 make up one's mind its molecular structure, which is described as T shaped. This is essentially a trigonal bipyramid that is missing two equatorial vertices. The Faxial–Br–Faxial angle is 172°, less than 180° because of LP–BP repulsions (Figure \(\PageIndex{two}\).ane).
Because lone pairs occupy more infinite effectually the central atom than bonding pairs, electrostatic repulsions are more important for lonely pairs than for bonding pairs.
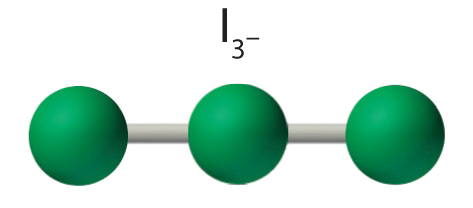
AX2E3 Molecules: I3 −
1. Each iodine atom contributes seven electrons and the negative charge one, so the Lewis electron structure is
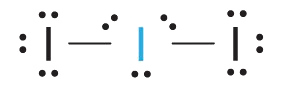
2. There are five electron groups nearly the cardinal atom in Ithree −, two bonding pairs and three alone pairs. To minimize repulsions, the groups are directed to the corners of a trigonal bipyramid.
3. With two bonding pairs and three lonely pairs, Iiii − has a full of five electron pairs and is designated as AX2E3. Nosotros must now make up one's mind how to accommodate the lone pairs of electrons in a trigonal bipyramid in a way that minimizes repulsions. Placing them in the axial positions eliminates 90° LP–LP repulsions and minimizes the number of ninety° LP–BP repulsions.
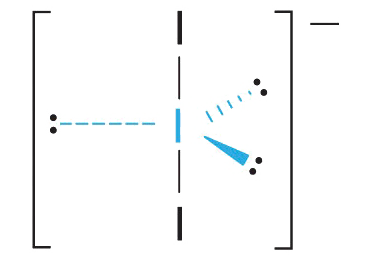
The three lone pairs of electrons have equivalent interactions with the three iodine atoms, so nosotros practice not expect whatever deviations in bonding angles.
4. With 3 nuclei and three lone pairs of electrons, the molecular geometry of I3 − is linear. This can be described as a trigonal bipyramid with three equatorial vertices missing. The ion has an I–I–I angle of 180°, as expected.
Half dozen Electron Groups
Six electron groups form an octahedron, a polyhedron made of identical equilateral triangles and six identical vertices (Effigy \(\PageIndex{2}\).)
AXsix Molecules: SF6
one. The key atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is
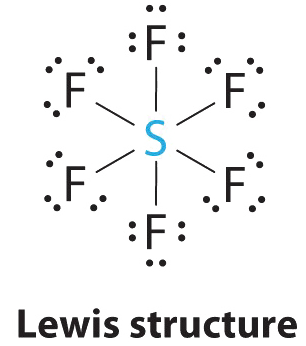
With an expanded valence, this species is an exception to the octet rule.
2. There are vi electron groups around the key atom, each a bonding pair. We see from Figure \(\PageIndex{2}\) that the geometry that minimizes repulsions is octahedral.
iii. With only bonding pairs, SF6 is designated as AX6. All positions are chemically equivalent, so all electronic interactions are equivalent.
iv. In that location are vi nuclei, so the molecular geometry of SF6 is octahedral.

AXfiveEast Molecules: BrFv
1. The central atom, bromine, has vii valence electrons, as does each fluorine, and so the Lewis electron construction is

With its expanded valence, this species is an exception to the octet rule.
2. There are 6 electron groups effectually the Br, five bonding pairs and 1 alone pair. Placing v F atoms around Br while minimizing BP–BP and LP–BP repulsions gives the following structure:
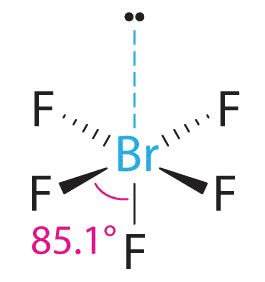
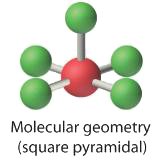
3. With five bonding pairs and one solitary pair, BrFv is designated equally AXvEast; it has a total of 6 electron pairs. The BrF5 construction has four fluorine atoms in a plane in an equatorial position and 1 fluorine atom and the solitary pair of electrons in the axial positions. We look all Faxial–Br–Fequatorial angles to exist less than 90° because of the lone pair of electrons, which occupies more space than the bonding electron pairs.
4. With five nuclei surrounding the fundamental atom, the molecular construction is based on an octahedron with a vertex missing. This molecular structure is foursquare pyramidal. The Faxial–B–Fequatorial angles are 85.1°, less than 90° considering of LP–BP repulsions.
AX4Eii Molecules: ICliv −
1. The central cantlet, iodine, contributes seven electrons. Each chlorine contributes 7, and there is a single negative charge. The Lewis electron structure is

ii. There are half dozen electron groups around the central atom, four bonding pairs and ii alone pairs. The structure that minimizes LP–LP, LP–BP, and BP–BP repulsions is
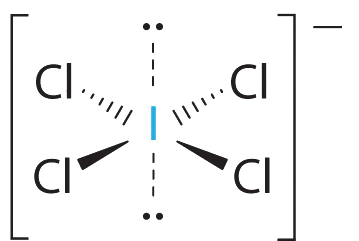
three. ICl4 − is designated as AX4East2 and has a total of six electron pairs. Although at that place are lone pairs of electrons, with 4 bonding electron pairs in the equatorial plane and the solitary pairs of electrons in the axial positions, all LP–BP repulsions are the same. Therefore, we do not await any deviation in the Cl–I–Cl bond angles.
4. With five nuclei, the ICl4− ion forms a molecular construction that is square planar, an octahedron with ii opposite vertices missing.
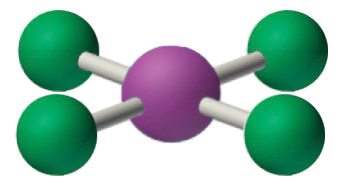
The relationship between the number of electron groups effectually a central atom, the number of lone pairs of electrons, and the molecular geometry is summarized in Effigy \(\PageIndex{half-dozen}\).
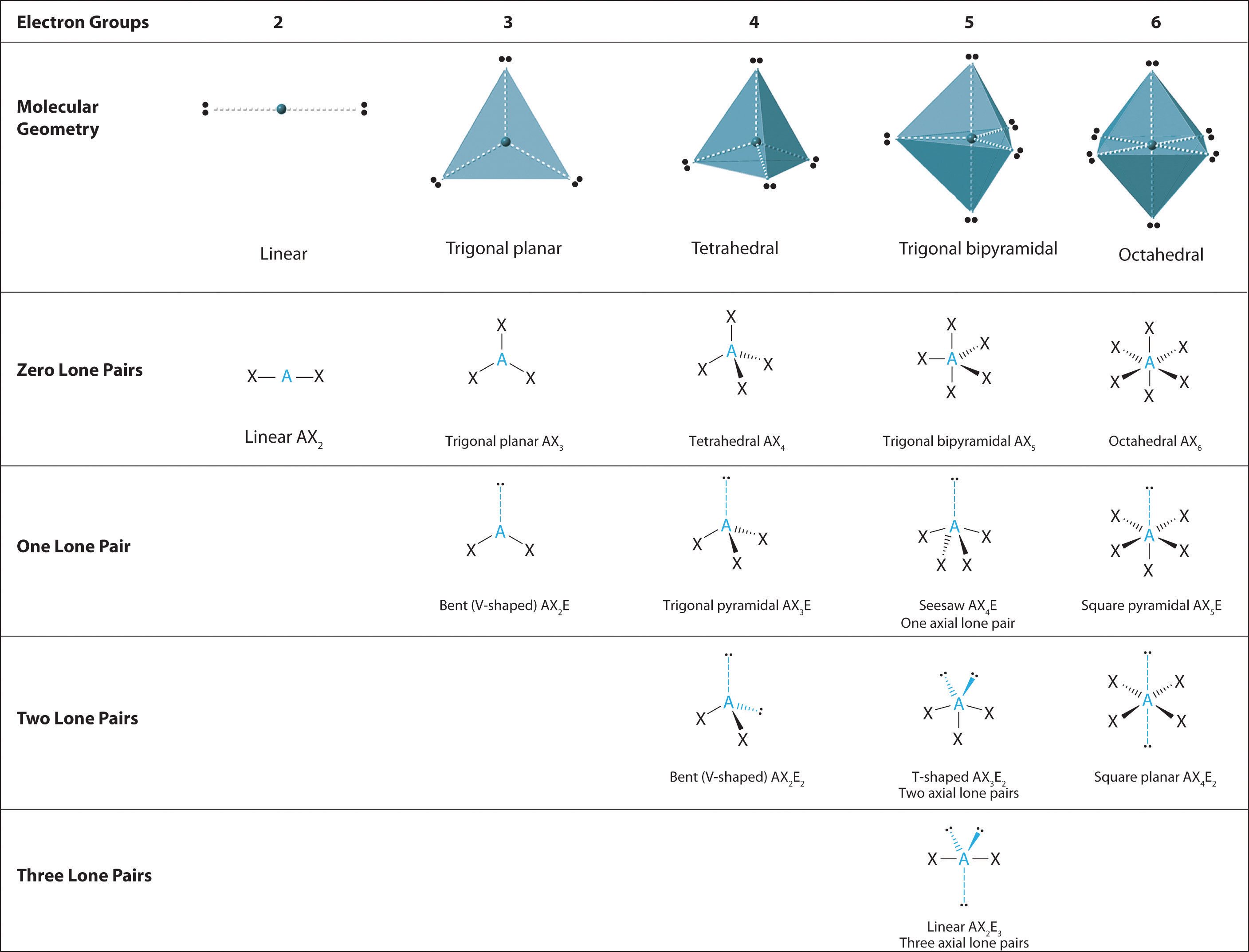
Figure \(\PageIndex{vi}\): Overview of Molecular Geometries
Instance \(\PageIndex{one}\)
Using the VSEPR model, predict the molecular geometry of each molecule or ion.
- PF5 (phosphorus pentafluoride, a catalyst used in certain organic reactions)
- H3O+ (hydronium ion)
Given: two chemic species
Asked for: molecular geometry
Strategy:
- Draw the Lewis electron structure of the molecule or polyatomic ion.
- Make up one's mind the electron grouping organisation around the cardinal atom that minimizes repulsions.
- Assign an AX chiliad E north designation; then place the LP–LP, LP–BP, or BP–BP interactions and predict deviations in bond angles.
- Describe the molecular geometry.
Solution:
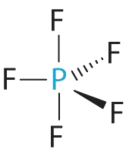
- A The central atom, P, has five valence electrons and each fluorine has vii valence electrons, so the Lewis structure of PFv is
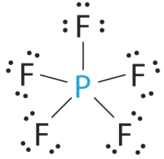
Figure \(\PageIndex{6}\)): (CC BY-NC-SA; anonymous) C All electron groups are bonding pairs, and then PF5 is designated every bit AX5. Detect that this gives a total of five electron pairs. With no alone pair repulsions, we do not expect any bond angles to deviate from the ideal.
D The PFfive molecule has five nuclei and no lone pairs of electrons, so its molecular geometry is trigonal bipyramidal.
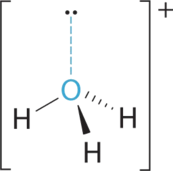
- A The central atom, O, has six valence electrons, and each H atom contributes one valence electron. Subtracting 1 electron for the positive charge gives a total of eight valence electrons, so the Lewis electron structure is
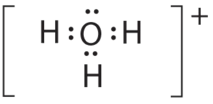
B There are four electron groups effectually oxygen, 3 bonding pairs and one lonely pair. Like NH3, repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron.
C With three bonding pairs and i lone pair, the structure is designated as AXthreeE and has a total of iv electron pairs (3 X and one E). We look the LP–BP interactions to cause the bonding pair angles to deviate significantly from the angles of a perfect tetrahedron.
D In that location are three nuclei and one lone pair, so the molecular geometry is trigonal pyramidal, in essence a tetrahedron missing a vertex. However, the H–O–H bond angles are less than the platonic angle of 109.5° because of LP–BP repulsions:
Practice \(\PageIndex{1}\)
Using the VSEPR model, predict the molecular geometry of each molecule or ion.
- XeOiii
- PF6 −
- NO2 +
- Answer a
-
trigonal pyramidal
- Reply b
-
octahedral
- Answer c
-
linear
Example \(\PageIndex{2}\)
Predict the molecular geometry of each molecule.
- XeF2
- SnCltwo
Given: two chemic compounds
Asked for: molecular geometry
Strategy:
Apply the strategy given in Example\(\PageIndex{1}\).
Solution:
- A Xenon contributes eight electrons and each fluorine seven valence electrons, and so the Lewis electron construction is

B There are five electron groups effectually the fundamental atom, two bonding pairs and three solitary pairs. Repulsions are minimized by placing the groups in the corners of a trigonal bipyramid.
C From B, XeFtwo is designated as AX2E3 and has a full of five electron pairs (two X and three East). With iii lone pairs near the primal cantlet, we tin arrange the ii F atoms in iii possible ways: both F atoms tin be axial, ane tin be axial and one equatorial, or both can be equatorial:
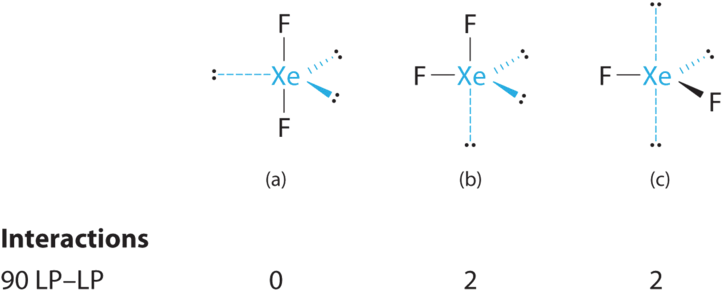
The structure with the lowest energy is the ane that minimizes LP–LP repulsions. Both (b) and (c) have two 90° LP–LP interactions, whereas construction (a) has none. Thus both F atoms are in the centric positions, like the two iodine atoms around the central iodine in Iiii −. All LP–BP interactions are equivalent, then we do not expect a deviation from an ideal 180° in the F–Xe–F bond angle.
D With two nuclei virtually the primal atom, the molecular geometry of XeFtwo is linear. It is a trigonal bipyramid with three missing equatorial vertices.
- A The tin can atom donates four valence electrons and each chlorine atom donates 7 valence electrons. With 18 valence electrons, the Lewis electron construction is
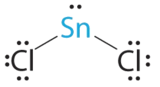
B In that location are three electron groups around the primal atom, 2 bonding groups and one lone pair of electrons. To minimize repulsions the three groups are initially placed at 120° angles from each other.
C From B we designate SnCl2 as AXtwoDue east. Information technology has a total of iii electron pairs, two X and ane East. Because the lone pair of electrons occupies more than space than the bonding pairs, we expect a subtract in the Cl–Sn–Cl bond bending due to increased LP–BP repulsions.
D With two nuclei around the central atom and ane lone pair of electrons, the molecular geometry of SnCl2 is bent, like SOtwo, just with a Cl–Sn–Cl bond angle of 95°. The molecular geometry tin exist described as a trigonal planar arrangement with one vertex missing.
Exercise \(\PageIndex{2}\)
Predict the molecular geometry of each molecule.
- SO3
- XeF4
- Answer a
-
trigonal planar
- Answer b
-
foursquare planar
Molecules with No Single Central Cantlet
The VSEPR model can be used to predict the construction of somewhat more than complex molecules with no unmarried central atom by treating them as linked AX m E n fragments. We will demonstrate with methyl isocyanate (CH3–N=C=O), a volatile and highly toxic molecule that is used to produce the pesticide Sevin. In 1984, big quantities of Sevin were accidentally released in Bhopal, India, when water leaked into storage tanks. The resulting highly exothermic reaction acquired a rapid increase in pressure level that ruptured the tanks, releasing large amounts of methyl isocyanate that killed approximately 3800 people and wholly or partially disabled virtually l,000 others. In addition, at that place was pregnant impairment to livestock and crops.
We can treat methyl isocyanate equally linked AX m E n fragments beginning with the carbon atom at the left, which is connected to iii H atoms and one North cantlet by single bonds. The four bonds around carbon mean that information technology must be surrounded by four bonding electron pairs in a configuration like to AX4. Nosotros tin can therefore predict the CHthree–N portion of the molecule to be roughly tetrahedral, like to methane:
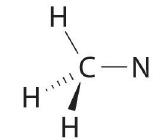
The nitrogen atom is connected to ane carbon past a single bail and to the other carbon by a double bail, producing a total of three bonds, C–N=C. For nitrogen to have an octet of electrons, it must also take a lonely pair:
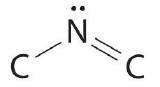
Because multiple bonds are not shown in the VSEPR model, the nitrogen is effectively surrounded by three electron pairs. Thus according to the VSEPR model, the C–N=C fragment should exist bent with an angle less than 120°.
The carbon in the –Due north=C=O fragment is doubly bonded to both nitrogen and oxygen, which in the VSEPR model gives carbon a total of ii electron pairs. The Northward=C=O bending should therefore be 180°, or linear. The three fragments combine to give the following construction:
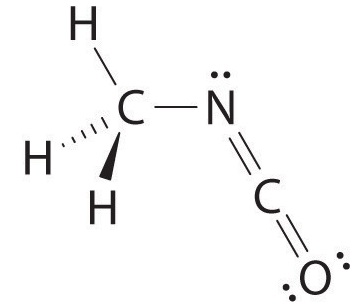
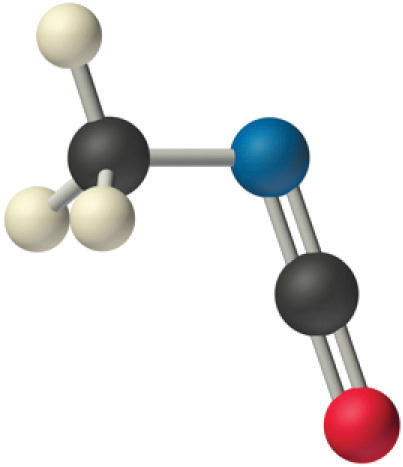
Certain patterns are seen in the structures of moderately complex molecules. For example, carbon atoms with four bonds (such equally the carbon on the left in methyl isocyanate) are generally tetrahedral. Similarly, the carbon atom on the right has two double bonds that are similar to those in COtwo, so its geometry, like that of CO2, is linear. Recognizing similarities to simpler molecules volition help you predict the molecular geometries of more complex molecules.
Example \(\PageIndex{3}\)
Use the VSEPR model to predict the molecular geometry of propyne (H3C–C≡CH), a gas with some coldhearted backdrop.
Given: chemical compound
Asked for: molecular geometry
Strategy:
Count the number of electron groups around each carbon, recognizing that in the VSEPR model, a multiple bail counts as a single group. Utilize Figure \(\PageIndex{3}\) to determine the molecular geometry around each carbon atom then deduce the construction of the molecule equally a whole.
Solution:
Considering the carbon atom on the left is bonded to four other atoms, we know that it is approximately tetrahedral. The adjacent two carbon atoms share a triple bond, and each has an additional single bond. Because a multiple bond is counted as a single bond in the VSEPR model, each carbon atom behaves as if it had 2 electron groups. This means that both of these carbons are linear, with C–C≡C and C≡C–H angles of 180°.
Exercise \(\PageIndex{3}\)
Predict the geometry of allene (H2C=C=CH2), a compound with narcotic properties that is used to brand more than complex organic molecules.
- Answer
-
The terminal carbon atoms are trigonal planar, the central carbon is linear, and the C–C–C angle is 180°.
Molecular Dipole Moments
Yous previously learned how to calculate the dipole moments of simple diatomic molecules. In more circuitous molecules with polar covalent bonds, the three-dimensional geometry and the compound'due south symmetry determine whether there is a net dipole moment. Mathematically, dipole moments are vectors; they possess both a magnitude and a management. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. If the individual bond dipole moments cancel one another, there is no internet dipole moment. Such is the case for COii, a linear molecule (Figure \(\PageIndex{8a}\)). Each C–O bond in COtwo is polar, nevertheless experiments show that the CO2 molecule has no dipole moment. Because the 2 C–O bond dipoles in CO2 are equal in magnitude and oriented at 180° to each other, they abolish. As a result, the CO2 molecule has no internet dipole moment fifty-fifty though it has a substantial separation of charge. In dissimilarity, the H2O molecule is non linear (Figure \(\PageIndex{8b}\)); it is bent in three-dimensional space, so the dipole moments do not cancel each other. Thus a molecule such as HiiO has a net dipole moment. We expect the concentration of negative charge to be on the oxygen, the more electronegative atom, and positive charge on the ii hydrogens. This charge polarization allows H2O to hydrogen-bond to other polarized or charged species, including other h2o molecules.
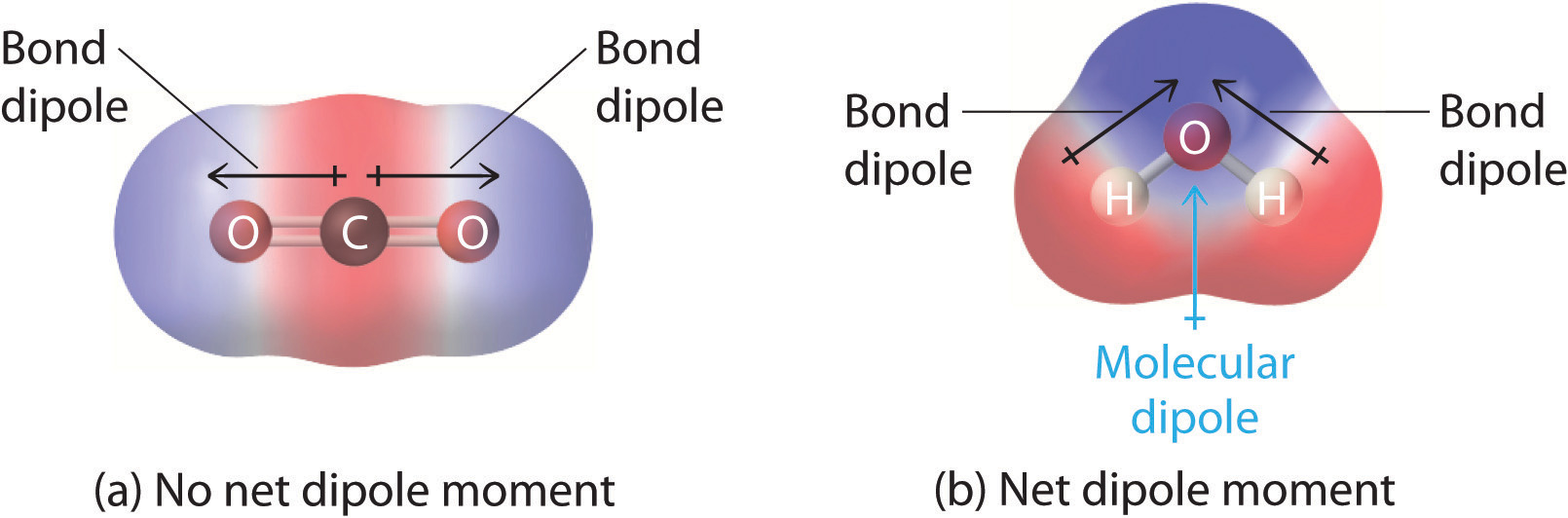
Other examples of molecules with polar bonds are shown in Figure \(\PageIndex{9}\). In molecular geometries that are highly symmetrical (nigh notably tetrahedral and square planar, trigonal bipyramidal, and octahedral), individual bond dipole moments completely cancel, and there is no cyberspace dipole moment. Although a molecule like CHCliii is best described equally tetrahedral, the atoms bonded to carbon are not identical. Consequently, the bond dipole moments cannot abolish 1 another, and the molecule has a dipole moment. Due to the system of the bonds in molecules that accept Five-shaped, trigonal pyramidal, seesaw, T-shaped, and square pyramidal geometries, the bond dipole moments cannot abolish one another. Consequently, molecules with these geometries ever have a nonzero dipole moment. Molecules with asymmetrical accuse distributions take a net dipole moment.

Example \(\PageIndex{4}\)
Which molecule(s) has a net dipole moment?
- \(\ce{H2S}\)
- \(\ce{NHF2}\)
- \(\ce{BF3}\)
Given: three chemical compounds
Asked for: internet dipole moment
Strategy:
For each three-dimensional molecular geometry, predict whether the bond dipoles cancel. If they do not, then the molecule has a net dipole moment.
Solution:
- The full number of electrons effectually the central atom, S, is eight, which gives 4 electron pairs. Two of these electron pairs are bonding pairs and two are lonely pairs, so the molecular geometry of \(\ce{H2S}\) is bent (Figure \(\PageIndex{half dozen}\)). The bond dipoles cannot cancel 1 another, and so the molecule has a net dipole moment.
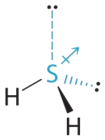
- Difluoroamine has a trigonal pyramidal molecular geometry. Considering there is ane hydrogen and two fluorines, and because of the lone pair of electrons on nitrogen, the molecule is not symmetrical, and the bond dipoles of NHF2 cannot cancel one another. This means that NHF2 has a cyberspace dipole moment. Nosotros wait polarization from the ii fluorine atoms, the most electronegative atoms in the periodic tabular array, to have a greater affect on the net dipole moment than polarization from the lone pair of electrons on nitrogen.
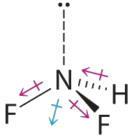
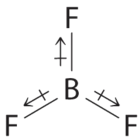
- The molecular geometry of BFthree is trigonal planar. Because all the B–F bonds are equal and the molecule is highly symmetrical, the dipoles cancel ane some other in iii-dimensional space. Thus BF3 has a net dipole moment of zero:
Exercise \(\PageIndex{4}\)
Which molecule(south) has a cyberspace dipole moment?
- \(\ce{CH3Cl}\)
- \(\ce{SO3}\)
- \(\ce{XeO3}\)
- Answer
-
\(\ce{CH3Cl}\) and \(\ce{XeO3}\)
Summary
Lewis electron structures give no information nearly molecular geometry, the arrangement of bonded atoms in a molecule or polyatomic ion, which is crucial to agreement the chemical science of a molecule. The valence-shell electron-pair repulsion (VSEPR) model allows united states of america to predict which of the possible structures is actually observed in nearly cases. It is based on the assumption that pairs of electrons occupy space, and the lowest-energy structure is the one that minimizes electron pair–electron pair repulsions. In the VSEPR model, the molecule or polyatomic ion is given an AX m E n designation, where A is the central atom, 10 is a bonded atom, Due east is a nonbonding valence electron group (unremarkably a lonely pair of electrons), and k and n are integers. Each group around the central atom is designated every bit a bonding pair (BP) or lone (nonbonding) pair (LP). From the BP and LP interactions we tin predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. From this we can draw the molecular geometry. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. A combination of VSEPR and a bonding model, such as Lewis electron structures, is necessary to understand the presence of multiple bonds.
Molecules with polar covalent bonds can have a dipole moment, an asymmetrical distribution of accuse that results in a tendency for molecules to align themselves in an applied electric field. Any diatomic molecule with a polar covalent bail has a dipole moment, merely in polyatomic molecules, the presence or absence of a cyberspace dipole moment depends on the structure. For some highly symmetrical structures, the individual bail dipole moments cancel one another, giving a dipole moment of nix.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/09._Molecular_Geometry_and_Bonding_Theories/9.2%3A_The_VSEPR_Model
0 Response to "do i include lone pairs in 3d drawings"
Kommentar veröffentlichen